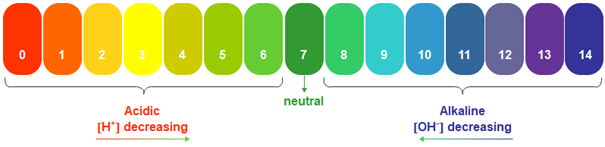
The pH value, commonly used for water measurements, is a measure of acidity and alkalinity, or the alkalis and bases present in a given solution.
The pH value is generally given on a numerical scale of 0-14. Here, the value 7 represents neutrality. The numbers on the scale increase as alkalinity increases, while the numbers on the scale decrease as acidity increases.
Each unit of change corresponds to a tenfold change in acidity or alkalinity. The pH is also equal to the negative logarithm of the hydrogen ion concentration or hydrogen ion activity.