The hardness of water is formed by dissolved salts. In natural waters, hardness is formed mainly from magnesium and calcium hydrogen carbonate and sulfate dissolved from the soil and / or aquifers.
The more salts dissolved in the water, the harder the water becomes. Since the composition of the geological subsoil varies greatly from region to region, a wide variety of water hardnesses and compositions are encountered in practice.
Other input pathways include dissolution by acidic components of precipitation or by nitric acid from nitrification resulting from agricultural fertilization.
-
Water Analyzers
- Devices
-
Parameters
-
Water hardness (Ca2+ and Mg2+)
- Total hardness (TH)
- Carbonate hardness (KH)
- Acid capacity (pH 4.3, KS 4.3) and p-value (pH 8.2, KS 8.2)
- Base capacity (KB82)
- Bromine (Br)
- Free Chlorine (HOCl)
- Total chlorine (CC)
- Chlorine dioxide (ClO2)
- Chromate & Chromium Cr(VI)
- Iron (II + III dissolved - Fe3O4)
- Silicate (SiO2 - dissolved)
- Ortho-Phosphat (P2O5)
- Polymere & Polyacrylate
- Monochloramine (NH2CL)
-
Water hardness (Ca2+ and Mg2+)
- TESTOMAT® - Accessories
- Spare Parts
- Service sets and maintenance cases
- Liquid chemistry
- Controls
- Electrodes and sensors
- Hand-held measuring instruments and analysis cases
- NeoTec World
- Clinical Solutions
- Blog
- Seminars & Webinars
- Ozone and UV technology
- Dosing pumps
- Gas Measuring Instruments
- Hygiene and Disinfection
- Water Meters
- Accessories
Filter products
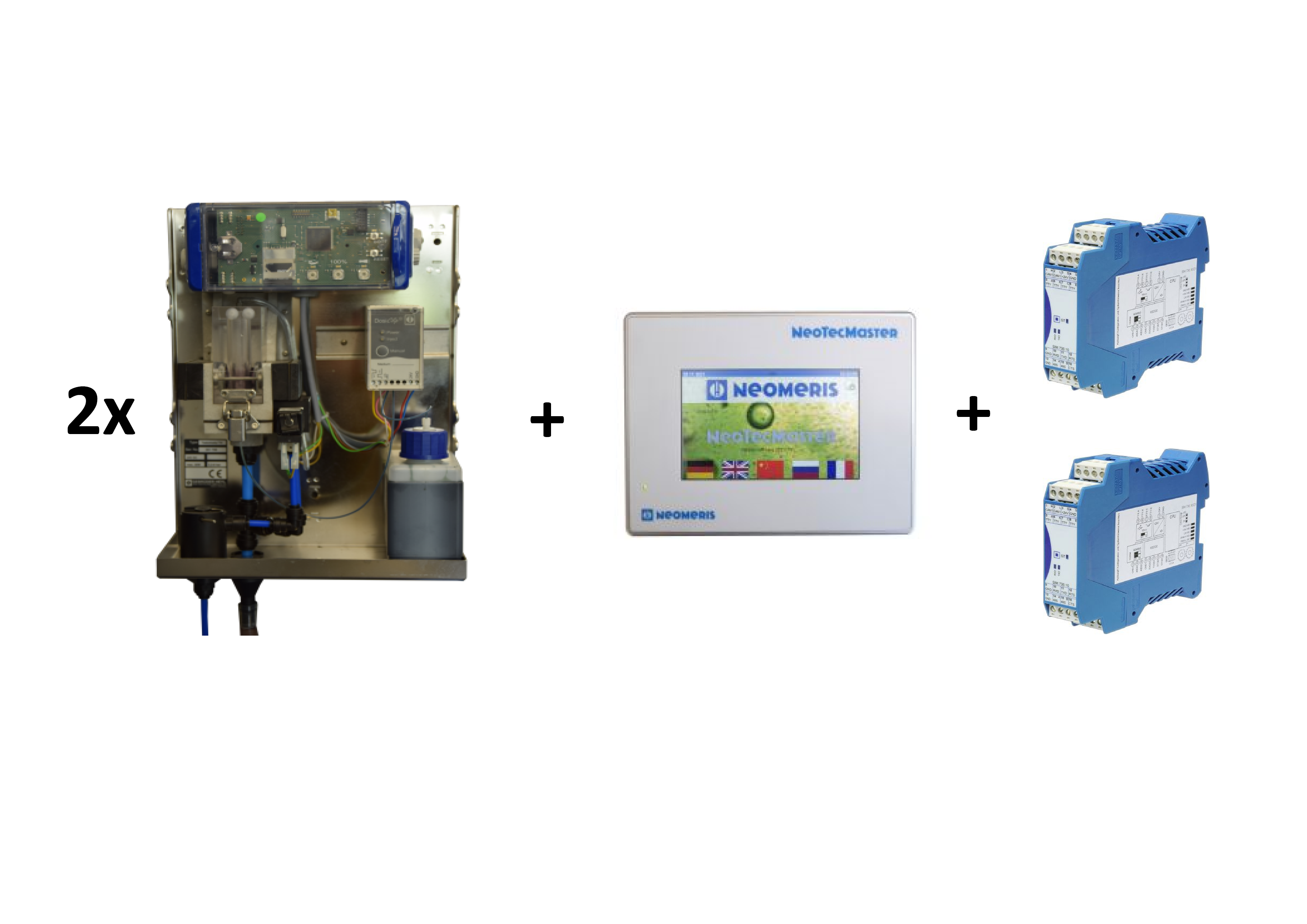
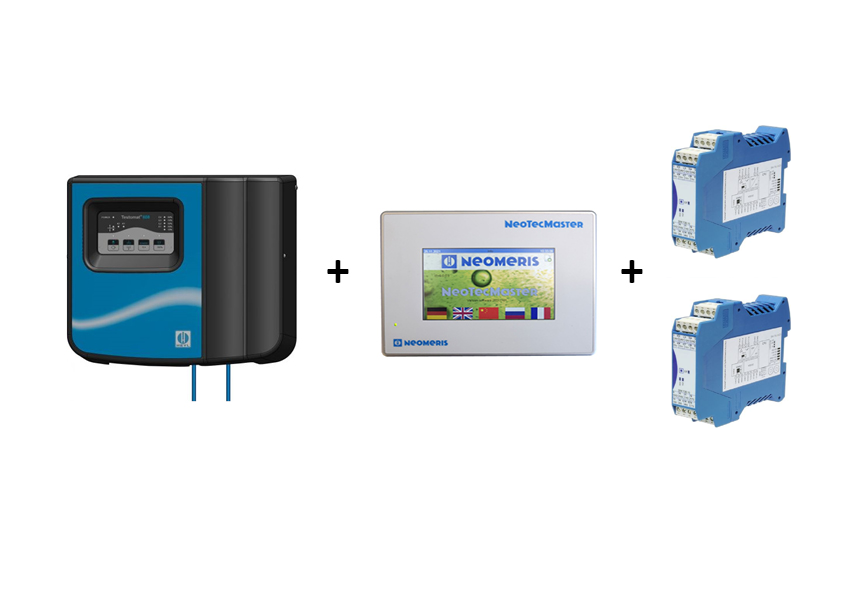
Die Integration einer Wasserhärtemessung ist ein wichtiger Bestandteil im Rahmen der industriellen Wasseraufbereitung. Hierdurch wird die Menge an gelöstem Calcium- und Magnesiumsalz in Wasser bestimmt. Das Vorhandensein von Salzen kann dazu führen, dass das Wasser hart wird und Kalkablagerungen entstehen, welche die Anlagentechnik beschädigen kann bzw. das herzustellenden Produkt negativ beeinflusst.
Um die Wasserhärte zu messen, gibt es verschiedene Testverfahren, von denen eines die Verwendung von Testomat-Systemen ist. Ein Testomat ist ein automatisches Messsystemen, das die Wasserhärte kontinuierlich und in Echtzeit messen kann.
Die Funktionsweise eines Testomaten basiert auf einem Farbindikator. Das zu messende Wasser fließt durch die Messkammer, in die durch eine Dosiclip Indikator hinzugefügt wird. Hierdurch reagiert das zu messende Wasser mit dem Farbindikator. Der Farbumschlag wird erfasst und in ein elektrisches Signal umgewandelt, welches den gemessenen Härtegrad im Display des Testomaten angezeigt.
Die Online-Analyse mittels Testomat ermöglicht eine genaue, zuverlässige und automatische Überwachung der Wasserhärte. Der Einsatz der Heyl Testomaten eignet sich daher in verschiedenen Bereichen: z.B. in der Lebensmittelindustrie oder in Schwimmbädern.